NuFLOW™ Microspheres and Investigator Partner™ Service
January 1997
Description: NuFLOW™ Microspheres and Investigator Partner™ Service provide a new method to measure regional organ blood flow. This process requires researchers to send their organ samples to Interactive Medical Technology (IMT) - Investigator Partner™ Service - (IPS ) where the number of microspheres in each sample are determined by flow cytometry and the results returned to the investigator in the form of an EXCEL™ spreadsheet. Although the use of flow cytometry has been validated for measuring regional blood flow, the entire process of sending samples to an independent laboratory has not been critically assessed in peer-review literature. Because of the numerous calls the FMRC receives concerning the NuFLOW™ Microspheres and Investigator Partner Service, we decided to test this new process against traditional radioactive microsphere methods.
Methods: Seven different colored NuFLOW™ microspheres 15 µm in diameter were obtained from Triton Technologies (San Diego, CA) in a kit costing $255. The specific colors used were red-high, violet-high, blue-high, red-med, orange-high, violet-med, and red-low. Each 2.1 ml bottle contained 50 million spheres in a saline solution, 0.01% Tween 80 and 0.01% Thimerosal as a bacteriostat. 1.25 million microspheres of each color were mixed with 1.3 million Ruthenium labeled microspheres and simultaneously injected into the left ventricle of an anesthetized and mechanically ventilated pig (15 kg). Two simultaneous reference blood samples were obtained from right and left femoral arterial lines. The animal was killed by anesthetic overdose and the heart and kidneys removed, diced into approximately 1 gram pieces and the radioactivity determined in a scintillation counter. The radioactive counts in each sample was corrected for background and decay. Blood flow to each piece (ml/min) was determined in duplicate from the two arterial reference blood samples. The organ and blood samples were frozen and stored until the radioactivity decayed to a safe level for shipment (3 months).
Thirty samples were selected on the basis of their initial radioactive counts to provide a wide range of flow values. Each sample was placed in an individual tube and sent to the IPS laboratory along with the reference blood samples ($10/sample, total $320). An electronic spreadsheet with the estimated blood flow for each sample (ml/min) was returned by email 2 weeks later ($60).
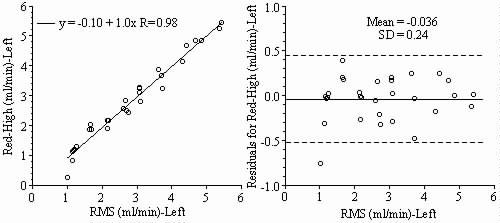
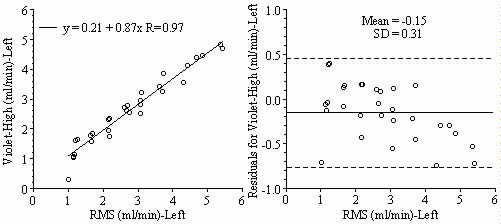
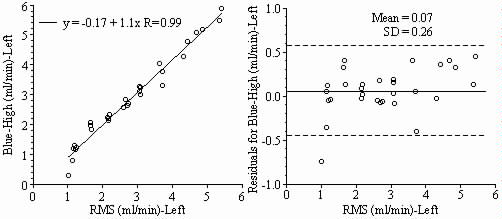
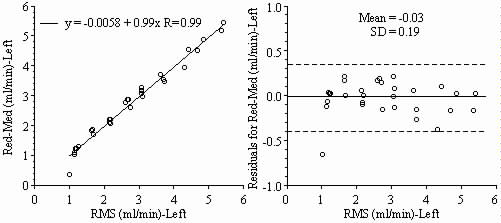
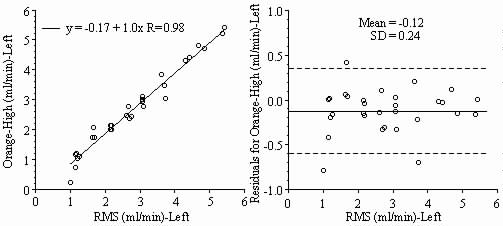
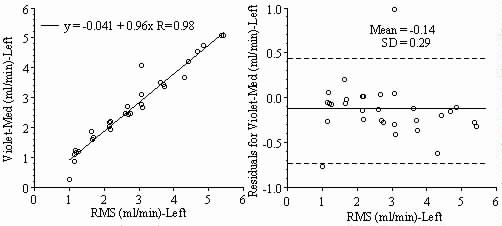
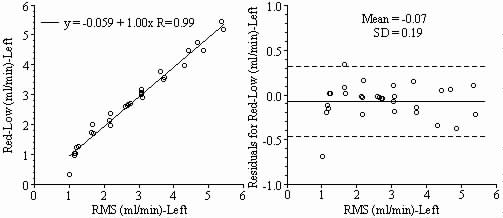
Results: Blood flows to each sample estimated from the NuFLOW™ Microspheres and Investigator Partner Service were compared to blood flows determined by the radioactive microsphere method. Correlation plots, Bland-Altman plots, and tables are presented below. Left and right arterial samples produced similar results. Only the results form the left reference sample are shown.