
- Potassium hydroxide pellets (224.4g KOH per 1000 ml H2O)
- 2% Tween-80™ (10 ml Tween-80 per 1000 ml potassium hydroxide)
- 100% isopropanol alcohol
- K2HPO4 powder (29.9 g K2HPO4 for 1000 ml buffer liquid)
- KH2PO4 powder (5.88 g KH2PO4 for 1000 ml buffer liquid)
- Organic solvent
Per Sample:
- 15 ml digestion solution
- 1.5 ml isopropanol
- 15 ml phosphate buffer
- organic solvent (Cellosolve™, Aldrich)
Preparation:
- Digestion solution: Dissolve 224.44 g KOH in 1000 ml H2O by constant stirring. Add 10 ml 2% Tween-80 when the liquid has cleared (stable for 24 hours).
- Phosphate buffer: Dissolve 5.88 g KH2PO4 in 200 ml and 29.9 g K2HPO4 in 800 ml H2O. Mix two solutions together.
Work Routine
- Place each tissue sample in a separate first stage of the filtering device.
- Place first stage in KOH bath or separate containers of KOH.
- Layer 1.5 ml of isopropanol over top of each sample and seal first stage (Parafilm, tinfoil, and plastic wrap work well).
- Heat samples to 60° C until digested (2 - 4 hr).
- Suction digestate through first filter stage by negative suction and rinse with 15 ml of buffer.
- Dry sample by centrifugation at 4000 rpm for 30 seconds.
- Put first stage of filtering device into the second stage and attach third stage to bottom.
- Add one-half volume of organic solvent to the first stage, vortex for 20 seconds and let rest for 5 min.
- Add remaining half of organic solvent to the first stage and vortex for 20 seconds.
- Centrifuge organic solvent to third stage of filtering device by centrifugation (4000 rpm for 30 secs.)
- Measure fluorescence in organic solvent.
Twenty-four (24) samples were digested using KOH and filtered with Poretic polycarbonate 10 µm filters. The fluorescent dye was extracted from each filter and the dye concentrations measured with our Perkin-Elmer LS50B. The fluorescent and radioactive signals from these samples defined the ratio of fluorescence to radioactivity expected in all other tissue samples.
Twenty-four (24) samples were digested and filtered per Institute for Surgical Research instructions. The fluorescent dye was extracted from each filter device per the protocol and the dye concentrations measured with our LS50B.
To see if the filter devices could be reused, the 24 filter devices were washed with soapy water and placed in an ultrasonicating bath for 60 minutes. Twenty-four (24) new tissue samples were digested and filtered per Institute for Surgical Research instructions, the fluorescent dye extracted from each filter device and the dye concentrations measured. This process was repeated five times so that 24 filter devices were each used a total of 6 times.
Results: Using the relationship between the Poretics filtered fluorescent signals and the radioactive counts as a standard for 100% recovery, it appears that all of the fluorescent microspheres were completely recovered by the eight Perkin Elmer filter devices (see graph below). The filter devices we tested were reusable, demonstrating 100% microsphere recovery after being used 6 times.
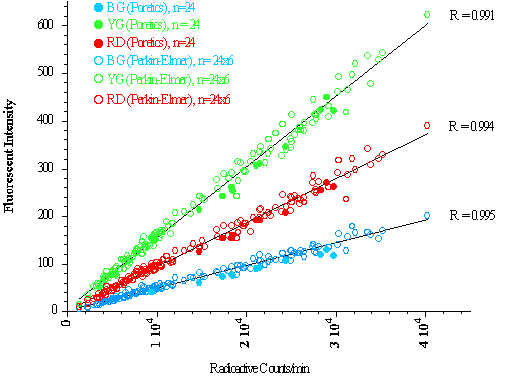
Conclusion: The Perkin Elmer filtering devices are an acceptable method to isolate fluorescent microspheres from solid tissue. Problems associated with the initial production run appear to have been resolved. Processing is more labor intensive than the Poretics filtration system for small numbers of samples. Significant time will be saved for larger sample numbers. The greatest advantage of the Perkin Elmer filtration system is that a tissue sample remains in a single container throughout, thereby minimizing the loss of microspheres during processing.
Although the filter devices are not meant to be reusable, we found that microsphere recovery was not compromised after being used at least 6 times. We did not continue to test them until they failed and therefore do not know how many times they can be used. We were able to determine if they failed because we had radioactive counts from the intact tissues. However, if only fluorescent microspheres are used in a study, the percent recovery will not be known and the competancy of the filters cannot be verified after multiple uses.
We received notification from Perkin-Elmer that the following kit has been discontinued. No replacement
appears to have been provided. (January 31, 2008)